Instructions: Sterling IRB
UH investigators engaged in industry-sponsored or PI-sponsored multisite clinical trials are encouraged to utilize the services of Sterling IRB (a commercial IRB) with whom UH has signed an IRB reliance agreement. UH IRB staff must review the PI’s Sterling submission(s) before the Sterling IRB is authorized to begin its review.
This page outlines the processes required of UH investigators wishing to rely on Sterling IRB. See https://www.sterlingirb.com/sterling-irb-handbook/ for the Sterling IRB Investigator handbook.
For research to be approved, Sterling IRB requires two separate kinds of submissions:
- A STUDY submission (the overall study that all sites will conduct)
- A SITE submission (each site is connected to the larger STUDY)
If the sponsor of the multi-site trial is a company, the company or CRO will likely be responsible for the STUDY submission. (The UH PI then applies as a SITE).
If the trial is PI-initiated, the PI will need to submit both the STUDY application and a SITE application.
STEPS:
- IMPORTANT: In all cases, contact the UH IRB Office to advise that you wish to request to rely on Sterling IRB. The UH IRB office will verify if the study is appropriate for reliance and confirm UH’s willingness to rely on the Sterling IRB for review. The IRB team can also provide further instruction for your submission.
- In all cases, the UH investigator must also submit an abbreviated “external” submission in ICON. You may start working on the ICON submission; however, you must wait to submit it until you receive the final protocol and formal approval documentation from Sterling. Follow these instructions for completing this submission.
- You will need access to Sterling’s IRB portal, “SilverLink.” To request access to SilverLink for the first time, contact Sterling at 770-690-9491.
If the larger “parent” STUDY has already been submitted to Sterling IRB by the Sponsor and UH is being added as a study SITE:
- You will need to add representatives from the UH IRB office as SITE CONTACTS. Please choose the “Site Monitor with Notifications” option for the following individuals:
- The consent form to be used at UH will require edits to meet Texas Medical Records
Privacy Act requirements. These edits, required by UH’s legal office, will require the PI to obtain written Sponsor approval before submitting to Sterling IRB. These changes are:
- The document’s HIPAA/confidentiality language must specifically state that the subject’s information may be released in electronic format, and
- The document’s HIPAA/confidentiality language must contain one of the following statements
regarding drug, alcohol, and substance abuse records:
- Special permission is required to release drug, alcohol, and substance abuse records,
HIV/AIDS-related information, genetic information and mental health information. These
kinds of records will not be used or disclosed in this study.
Or
Special permission is required to release drug, alcohol, and substance abuse records, HIV/AIDS-related information, genetic information and mental health information. With the exception of ________________ (previously mentioned on page ___), these kinds of records will not be used or disclosed in this study.
Required changes to the standard Sterling template consent language are here. If a sponsor’s consent form language is utilized, similar changes must be made and reviewed by the UH IRB office prior to submission to Sterling. As stated above, please ensure written permission is obtained from the sponsor prior to upload.
- Special permission is required to release drug, alcohol, and substance abuse records,
HIV/AIDS-related information, genetic information and mental health information. These
kinds of records will not be used or disclosed in this study.
- If you are a first time SITE PI with Sterling IRB, Sterling will require additional information from you (for example, CV, applicable licenses, DEA certificate etc.) be uploaded.
- All SITE-specific forms (consent and recruitment documents on UH letterhead and with UH investigator information) must be uploaded to the SITE application.
If the STUDY is PI-initiated, you will also need to submit the larger “parent” STUDY to Sterling IRB before any study SITES (including UH) may be created.
For a STUDY submission,
- The two individuals named above:
In addition to being named study contacts, must also be added to the STUDY submission as collaborators. Select the Collaborator link located at the top of each form and fill out the requested information:

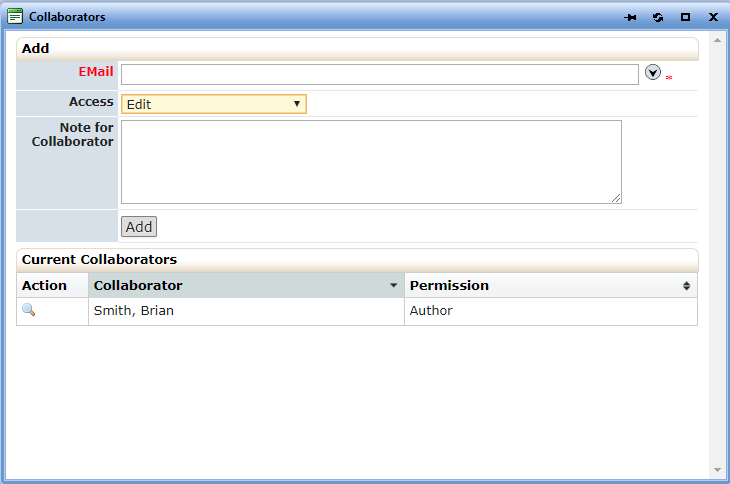
- See 2(a) and 2(b), above regarding HIPAA language that must be added for any studies you submit. Other sites must also use this language if you are the overall PI for all sites.
- All STUDY-specific documents must be uploaded; these can include templates for other
sites to use (consent forms, advertisements, scripts, case reporting forms), but the
content must be approved by Sterling IRB.
- Copies of letters resulting from recent FDA inspections (contact the IRB office for help)
Final notes:
- Once you have submitted your STUDY and/or SITE in the SilverLink system, contact the UH IRB Office. The UH IRB office must do a cursory review of the STUDY/SITE forms, consent form(s), and attachments for completeness, consistency, and accuracy. The IRB office will also review for UH-required language and answer any institutional questions (for example, whether the FDA has conducted a visit in the past 5 years).
- Once the UH IRB Office provides institutional sign-off in the Silverlink system, Sterling IRB will conduct a pre-review and send back any administrative corrections needed.
- Sterling staff route the submission for Sterling IRB review.
- Once all Sterling IRB requirements have been addressed, Sterling IRB notifies you and the UH IRB office of approval through Silverlink. This does not mean you may begin your research; an additional step is required.
- Once the STUDY/SITE is approved by Sterling, you must attach the following Sterling-approved
documents to your ICON external protocol submission:
- Sterling IRB approved protocol
- Sterling IRB-approved study instruments
- Sterling IRB-approved consent/assent form(s) to be used at UH
- Sterling IRB-approved recruitment materials to be used at UH
- Add the designated UH ancillary reviewers to your ICON external IRB protocol and submit.
- Once the ICON submission contains all required documents and has been signed by the appropriate ancillary reviewer, the UH IRB provides a final acknowledgment letter through the ICON system to rely on Sterling. Once this acknowledgment letter is received, UH engagement in the research may begin.
- Please note that all post-approval approvals and documentation (modifications, continuing reviews, etc.) must also be added to the ICON protocol following Sterling approval so UH can maintain oversight of the study conduct. All unanticipated events involving risk to subjects or others must be submitted to UH concurrent with the submission to Sterling, as further institutional review may be required.