Hydrogen a Sustainable Alternative to Fossil Fuels
“In a perfect world, we would be able to use sunshine, which is everywhere, and water, which covers 75 percent of the globe, to produce hydrogen for use as fuel,” said Randy Thummel, John and Rebecca Moores Professor of Chemistry in the College of Natural Sciences and Mathematics.
Hydrogen: An Alternative to Fossil Fuels
 Thummel received a 3-year, $570,000 grant from the Department of Energy. This grant
will fund his on-going efforts to develop a catalyst that can use the energy from
sunlight to break down water into hydrogen and oxygen.When water is broken down, or decomposed, it produces hydrogen and oxygen. When hydrogen
is combusted, it uses oxygen to produce water and energy. This cycle of water decomposition
and hydrogen combustion promises a sustainable alternative to fossil fuels.
Thummel received a 3-year, $570,000 grant from the Department of Energy. This grant
will fund his on-going efforts to develop a catalyst that can use the energy from
sunlight to break down water into hydrogen and oxygen.When water is broken down, or decomposed, it produces hydrogen and oxygen. When hydrogen
is combusted, it uses oxygen to produce water and energy. This cycle of water decomposition
and hydrogen combustion promises a sustainable alternative to fossil fuels.
Currently, a limited number of demonstration cars that use hydrogen as a fuel are operating in California. For this technology to be practical, a number of hurdles need to be overcome, one major challenge being hydrogen production.
Current Hydrogen Production Methods are Limited
Thummel recently was awarded a three-year, $570,000 grant from the Department of Energy to fund his ongoing efforts to develop a catalyst that can use the energy from sunlight to break down water into hydrogen and oxygen.
“The critical question for hydrogen to serve as a transportation fuel is where do you get the hydrogen?” he said “Right now, the hydrogen comes from two sources. The first is through thermal cracking of hydrocarbons, which means that you take gasoline and convert it to hydrogen. The other way is through the electrolysis of water, the energy cost of which exceeds the value of the energy that you get from burning the hydrogen.”
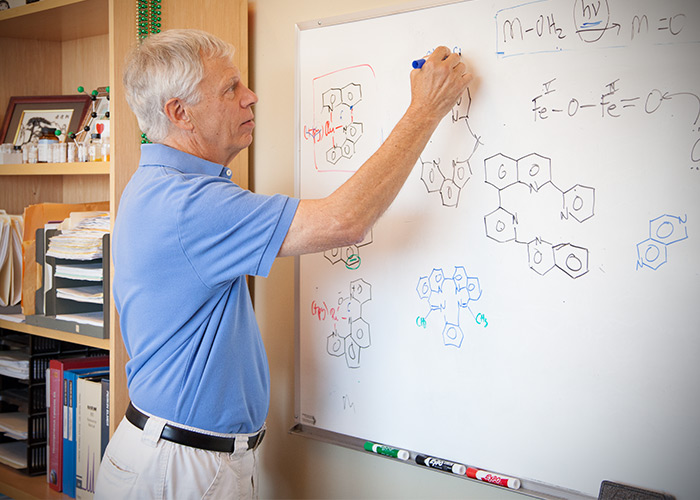
Thummel, as a synthetic organic chemist, develops catalysts which can facilitate chemical reactions without undergoing any permanent change in the catalyst. This means the catalyst can be used over and over again.
“Obviously, breaking down water using sunlight does not occur without a catalyst. Otherwise, the water in our oceans would be bubbling away,” he said. “We’d like to create a catalyst that can absorb sunlight, creating a higher energy state. This higher energy state can then be used to split a water molecule into hydrogen and oxygen.”
Challenge is Finding Catalysts that Produce Hydrogen and Oxygen
Scientists have been trying to develop efficient ways to break down water for a long time. On the surface, the chemistry is simple: break the bonds between oxygen and hydrogen in order to form bonds between two hydrog.
“At first, this looks like a piece of cake,” he said. “Unfortunately, getting catalysts to do this sort of chemistry is tough.”
Currently, there are catalysts that work with light to produce hydrogen, and catalysts that make oxygen but don’t work with light. The challenge is finding a way to form both hydrogen and oxygen in the same reaction so that everything balances out,” he said.
Thummel’s research is focused on combining several methods, which he thinks may be the key to developing this balanced reaction.
“If we can use water and sunlight in a constructive way, that is a huge step forward,” he said. To quote Jules Verne from the novel Mysterious Island: “Water will be the coal of the future.”
- Rachel Fairbank, College of Natural Sciences and Mathematics