Faculty Profile
 Ping Yi
Ping Yi
Assistant Professor
Department of Biology and Biochemistry
Research Division: Biochemistry (Primary)
Office: Science & Engineering Research Center, 3021
Contact: pyi3@central.uh.edu - 713-743-7230
Education: Ph.D., University of Rochester
Dr. Ping Yi’s lab research has been focused on molecular regulation of oncogene activation in cancer, with a particular focus on post-translational modification and signal transduction pathways, and nuclear receptor structural studies. Current studies aim to explore the role of non-proteolytic ubiquitination in prostate and lung cancer development and progression. The lab employs diverse tools for the studies, including transgenic mouse models, xenograft tumor models and a variety of molecular biology and cell biology tools. Another interest of the lab is to understand the structure and function of nuclear receptor-coregulator complexes. They are the first group to use cryo electron microscopy (Cryo-EM) to determine the quaternary structures of DNA-bound estrogen receptor alpha and androgen receptor with Steroid Receptor Coactivator (SRC-3) and a secondary coactivator (p300) complexes. Combining cryo-EM structural approach and biochemical analysis, they have elucidated a series of nuclear receptor complex structures.
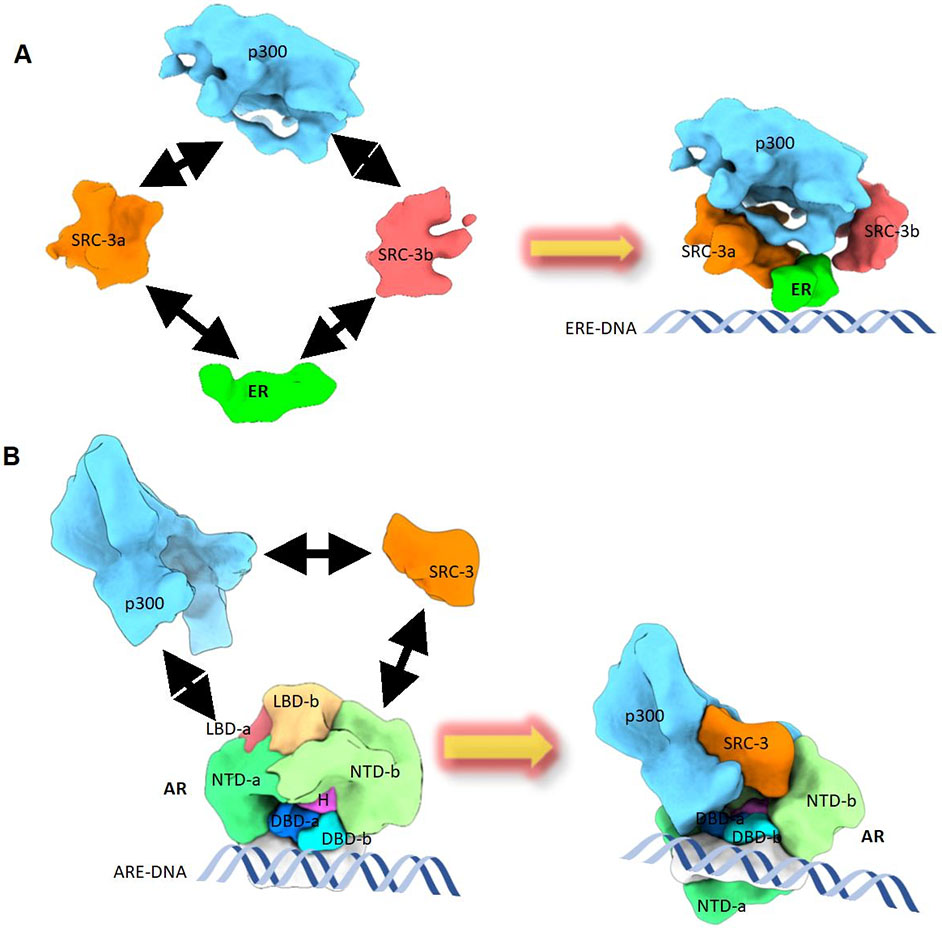
Figure: CryoEM structures reveal different assembly mechanisms for estrogen receptor and androgen receptor to recruit coactivators to activate target gene transcription. (A) The structural assembly of DNA-bound ERa, with coactivators (Steroid Receptor Coactivator-3 (SRC-3) and p300) complex. ERa recruits two SRC-3s (-a and -b) and the two SRC-3s interact with different regions of p300 to bring in p300 to the ERa binding site. (B) The structural assembly of DNA-bound AR, SRC-3, and p300 complex. AR interacts directly with one SRC-3 and one p300. The AR NTD-b (N-terminal domain-b) is solely responsible for SRC-3 recruitment. Both AR NTDs are major contributors for p300 recruitment. Small areas of LBDs (Ligand-binding domain) also interact with p300. The arrows indicate the interaction between different proteins.
- Yi, P., Yu, X., Wang, Z., O'Malley, B. W. Steroid receptor-coregulator transcriptional complexes: new insights from CryoEM. Essays Biochem. (2021) 65, 857–866.
- Yu, W., Singh, R., Wang, Z., O'Malley, B. W., Yi, P. The E3 ligase TRAF4 promotes IGF signaling by mediating atypical ubiquitination of IRS-1. J. Biol. Chem. (2021) 296, 100739.
- Yu, X*., Yi, P*., Hamilton, R. A., Shen, H., Chen, M., Foulds, C. E., Mancini, M. A., Ludtke, S. J., Wang, Z., O’Malley, B. W. Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator Complexes. Mol. Cell (2020) 79, 812-823. (*equal contribution)
- Panigrahi, A.K., Foulds, C.E., Lanz, R.B., Hamilton, R.A., Yi, P., Lonard, D.M., Tsai, M.J., Tsai, S.Y., O'Malley, B.W. SRC-3 Coactivator Governs Dynamic Estrogen-Induced Chromatin Looping Interactions during Transcription. Mol. Cell (2018) 70, 679-694.
- Singh, R., Karri, D., Shen, H., Shao, J., Dasgupta, S., Huang, S., Edwards, D.P., Ittmann, M.M., O'Malley, B.W., Yi, P. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J Clin. Invest. (2018) 128, 3129-3143.
- Dasgupta, S., Rajapakshe, K., Zhu, B., Nikolai, B. C., Yi, P., Putluri, N., Choi, J. M., Jung, S. Y., Coarfa, C., Westbrook, T. F., Zhang, X. H., Foulds, C. E., Tsai, S. Y., Tsai M. J., O'Malley, B. W. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature (2018) 556, 249-254.
- Yi, P., Wang, Z., Feng, Q., Chou C. K., Pintilie, G. D., Shen, H., Foulds, C. E., Fan, G., Serysheva, I., Ludtke, S. J., Schmid, M. F., Hung, M. C., Chiu, W., O'Malley, B. W. Structural and Functional Impacts of ER Coactivator Sequential Recruitment. Mol. Cell (2017) 67:733-743.
- Yi, P., Wang, Z., Feng, Q., Pintilie, G. D., Foulds, C. E., Lanz, R. B., Ludtke, S. J., Schmid, M. F., Chiu, W., O’Malley, B. W. The Structure of a Biological Active Estrogen Receptor-Coactivator Complex on DNA. Mol. Cell (2015) 57, 1047-1058.
- He, B., Lanz, R. B., Fiskus, W., Geng, c., Yi, P., Hartig, S. M., Rajapakshe, K., Shou, J., Wei, Li., Shah, S. S., Foley, C., Chew, S. A., Edunun, V. K., Bedoya, d. J., Feng, Q., Frolov, a., Weigel, N. L., Hilsenbeck, S. G., Palzkill, T. G., Ittmann, M. M., Song, Y., Coarfa, C., O’Malley, B. W., Mitsiades, N. GATA2 facilitates steroid receptor coactivator (SRC) recruitment to the androgen receptor (AR) complex in prostate cancer cells. Proc. Natl. Acad. Sci USA (2014) 111, 18261-18266.
- Yi, P., Xia, W., Wu, R. C., Lonard, D. M., Hung, M. C., O'Malley, B. W. SRC-3 Coactivator Regulates Cell resistance to Cytotoxic Stress via TRAF4-mediated p53 De-stabilization. Genes & Dev. (2013) 27, 274-287.
Honors & Awards
- 2020, Department of Defense Prostate Cancer Research Program Idea Expansion Award
- 2015-2019, Department of Defense Prostate Cancer Research Program Idea Award
- 2004-2007, Department of Defense Breast Cancer Research Program Postdoctoral Award
- 2005, Endocrine Society Quest Diagnostics Young Investigator Travel Grant
- 2004, Second Prize, Oral Scientific Presentation, SCBA Texas Chapter Annual Symposium
- 1999-2001, Department of Defense Breast Cancer Research Program Pre-doctoral Traineeship Award
- 1999, Travel Award of the 82nd Annual Meeting of the Endocrine Society
- 1994-1995, Yilida Scholarship, Second Prize, Chinese Academy of Sciences
- 1992-1993, People's scholarship, Third Prize, Wuhan University, China
Organizations, Outreach, Boards, Memberships
- 2017-present, American Association of Cancer Research member
- 1999-2014, The Endocrine Society member