Faculty Profile
 Thomas A. Albright
Thomas A. Albright
Professor Emeritus
Department of Chemistry
Office: Fleming, 218
Contact: talbright@uh.edu - 713-743-3270
Education: Ph.D., University of Delaware, 1975; B.S., North Dakota State University, 1970
A study of reaction mechanisms and rearrangements in inorganic, organometallic and solid state chemistry by molecular orbital theory.
Our research group carries out molecular orbital calculations at several levels to investigate static and dynamic properties of discrete compounds, as well as solid state materials. These calculations vary in complexity from the ab initio to extended Hückel levels. There exists a wide variety of analytical methods to view the results from these calculations.
 In the molecular area, a common theme that we are exploring is the mechanisms which
form carbon-carbon bonds in transition metal complexes. Some examples include the
trimerization of acetylene via Ta(OR)3 and CoCp based catalysts, the acetylene metathesis reaction and the dimerization
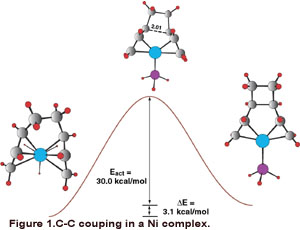
of butadiene with Ni(O). An example of a step in this catalytic cycle is shown in
Figure 1. We also are developing a series of orbital symmetry rules for the coupling
of unsaturated ligands coordinated to a metal, electrocyclic ring closure reactions
in polyene-MLn complexes and haptotropic rearrangements. The calculations in our group are arried
out at the Hartree-Foch level with some perturbation theory, CASSCF, QCISD or coupled
cluster techniques) or more frequently with a hybrid density functional.
In the molecular area, a common theme that we are exploring is the mechanisms which
form carbon-carbon bonds in transition metal complexes. Some examples include the
trimerization of acetylene via Ta(OR)3 and CoCp based catalysts, the acetylene metathesis reaction and the dimerization
of butadiene with Ni(O). An example of a step in this catalytic cycle is shown in
Figure 1. We also are developing a series of orbital symmetry rules for the coupling
of unsaturated ligands coordinated to a metal, electrocyclic ring closure reactions
in polyene-MLn complexes and haptotropic rearrangements. The calculations in our group are arried
out at the Hartree-Foch level with some perturbation theory, CASSCF, QCISD or coupled
cluster techniques) or more frequently with a hybrid density functional.
 In the solid state area, we primarily use tight binding calculations with an extended
Hückel type Hamiltonian. We have looked at a number of compounds which are ferroelectric.
Our interest here is to derive an electronic rationale for the distortion which occurs
upon going to the ferroelectric state, as well as the mechanism for this deformation.
Some examples include TlGaSe2, TlBiTe2, and the Aurivillius phases. We have also examined the electronic sources of Te-Te
bond formation in CuTe, K2Cu5Te5, etc. We also have an ongoing collaboration within the Texas Center for Superconducting
at the University of Houston to examine the bonding and mechanisms for superconductivity
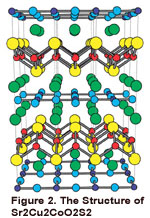
in new materials. An example of one compound that has been investigated is shown in
Figure 2.
In the solid state area, we primarily use tight binding calculations with an extended
Hückel type Hamiltonian. We have looked at a number of compounds which are ferroelectric.
Our interest here is to derive an electronic rationale for the distortion which occurs
upon going to the ferroelectric state, as well as the mechanism for this deformation.
Some examples include TlGaSe2, TlBiTe2, and the Aurivillius phases. We have also examined the electronic sources of Te-Te
bond formation in CuTe, K2Cu5Te5, etc. We also have an ongoing collaboration within the Texas Center for Superconducting
at the University of Houston to examine the bonding and mechanisms for superconductivity
in new materials. An example of one compound that has been investigated is shown in
Figure 2.
- Postdoctoral Fellow, Cornell University: 1975-1977
- Camille and Henry Dreyfus Teacher-Scholar: 1979-1984
- Alfred P. Sloan Research Fellow: 1982-1984
- Editorial Board Organometallics: 1988-1991
- H. Tang, D. M. Hoffman, T. A. Albright, H. Deng and R. Hoffmann, "Ni(P-t-Bu)6 and Ni(SiH2)6 Are IsolobalRelated to In[Mn(CO)4]52-, and Have 16-Electron Counts," Angew. Chem., 105, 1682 (1993).
- S. Seong, T. A. Albright, X. Zhang and M. Kanatzidis, "Te-Te Bonding in Copper Tellurides" J. Am. Chem. Soc., 116, 7287 (1994).
- Y. Oishi, T. A. Albright and H. Fujimoto, "Fluxionality and Bonding in (BH4)Mn(CO)4 and (BH4)Cu(PH3)2", Polyhedron, 14, 2603 (1995).
- W. J. Zhu, P. H. Hor, A. J. Jacobson, G. Crisi, T. A. Albright, S.-H. Wang, and T. Vogt, "A2Cu2CoO2S 2 (A=Sr, Ba), A Novel Example of a Square-planar Layer", J. Am. Chem. Soc., 119, 12398 (1997).
- C. Soubra, F. Chan and T. A. Albright, "Hydrogen Site Exchange in CpIr(PR3)H+3 Complexes", Inorg. Chim. Acta, 272, 95 (1998).
- J. Hardesty, J. B. Koerner, T. A. Albright and G.-Y. Lee, "A Theoretical Study of the Acetylene Trimerization with CpCo, J. Am. Chem. Soc., 121, 6055 (1999).
- S. Suh, J. H. Hardesty, T. A. Albright and D. M. Hoffman, "Synthesis and Structures of Gallium Alkylthiolate Compounds", Inorg. Chem., 38, 1627 (1999).
- Jon H. Hardesty, Thomas A. Albright, and Samia Kahlal, "A Theoretical Investigation of Electrocyclic Ring Closure Reactions in Bis-(aryloxy)bis-(h2-iminoacyl)zirconium and Isoelectronic Complexes", Organometallics,19,4159 (2000).
- C. Soubra, Y. Oishi, T. A. Albright and H. Fujimoto, "Intramolecular Rearrangements in Six-coordinate Ru and Fe Dihydride Complexes", Inorg. Chem., 40, 620 (2001).