Potential Applications Could Lead to a Net-Zero World
In a world increasingly concerned about the environmental and geopolitical implications of fossil fuel usage, nuclear energy has resurfaced as a subject of great interest. Its ability to generate electricity at scale without greenhouse gas emissions holds promise as a sustainable clean energy source that could bridge society’s transition away from fossil fuels to a net-zero future. However, nuclear power generation does produce radioactive waste. The safe management of nuclear waste remains a crucial challenge that must be addressed to gain public confidence in this transformative power solution.

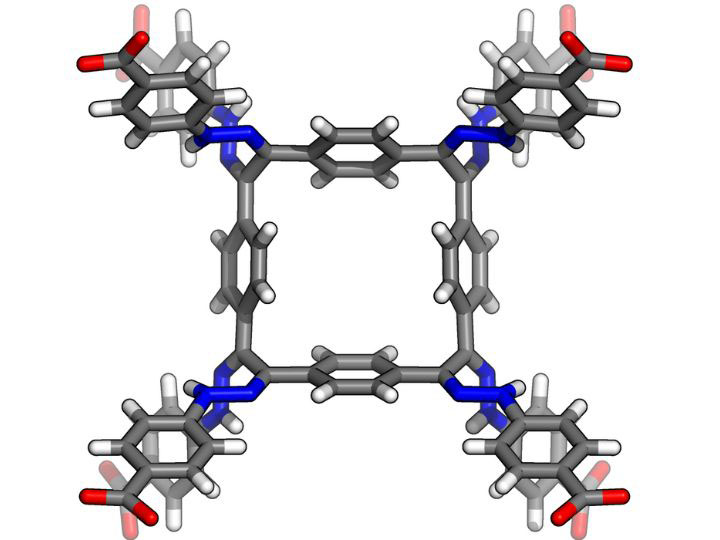
Now, a team of University of Houston researchers in the Department of Chemistry has come up with an innovative solution for nuclear waste management: molecular crystals based on cyclotetrabenzil hydrazones. These crystals, which are based on a groundbreaking discovery made by the team in 2015, are capable of capturing iodine — one of the most common radioactive fission products — in aqueous and organic solutions, and on the interface between the two.

“This last point is particularly salient because iodine capture on interfaces could prevent the iodine from reaching and damaging the specialized paint coatings used in nuclear reactors and waste containment vessels,” said Ognjen Miljanic, professor of chemistry and corresponding author of the paper detailing the breakthrough in Cell Reports Physical Science. This work was funded by the National Science Foundation.
These crystals exhibit an astonishing iodine uptake capacity, rivaling that of porous metal-organic frameworks (MOFs) and covalent organic frameworks (COFs), which were previously deemed the pinnacle of iodine capture materials.
Alexandra Robles, the first author of the study and a former chemistry doctoral student who based her dissertation on this research, was working with the crystals in Miljanic’s lab when she made the discovery. Her interest in finding a solution for nuclear waste led Robles to investigate using crystals to capture iodine.
“She ended up capturing iodine on the interface between the organic and water layers, which is an understudied phenomenon,” said Miljanic, who added that this exceptional feature provides a crucial advantage. “When the material is deposited between the organic and aqueous layer, it essentially stops the transfer of iodine from one layer to another.”

Not only does this process preserve integrity of reactor coatings and enhance containment, but the captured iodine could also then be moved from one area to another. “The idea here is that you capture it at a place where it's difficult to manage, and then you release it at a place where it's easy to manage,” said Miljanic, who is on the faculty of the UH College of Natural Sciences and Mathematics.
The other benefit of this catch-and-release technology is that the crystals can be reused. “If the pollutant just sticks to the regent, the whole thing has to be thrown away,” he said. “And that increases waste and economic loss.”
Of course, all of these great potentials still need to be tested in practical applications, which has Miljanic thinking of the next steps.
Molecules, Crystals and Octopi, Oh My!
Miljanic’s team creates these tiny organic molecules containing only carbon, hydrogen and oxygen atoms using commercially available chemicals.
Each crystal is a ring-shaped structure with eight linear piece emanating from it, which has led the research team to nickname it “The Octopus.”
“They are quite easy to make and can be produced at a large scale from relatively inexpensive materials without any special protective atmosphere,” said Miljanic.
He estimated that he can currently produce these crystals at the cost of about $1 per gram in an academic lab. In an industrial setting, Miljanic believes the cost would drop significantly.
These hungry little crystals are very versatile and can capture more than iodine. Miljanic and his team have used some of them to capture carbon dioxide, which would be another great step toward a cleaner, more sustainable world. In addition, “The Octopus” molecules are closely related to those found in materials used to make lithium-ion batteries, which opens the door to other energy opportunities.
“This is a type of simple molecule that can do all sorts of different things depending on how we integrate it with the rest of any given system,” Miljanic said. “So, we’re pursuing all those applications as well.”
He is excited by the multitude of potential offered by the crystals and looking forward to exploring practical applications. His next goal is to find a partner who will help the scientists explore different commercial aspects.
Until then, the researchers are planning to further explore the kinetics and behaviors of the crystal structures to make them even better.
- Rashda Khan, University Media Relations