 Most rocks are mixtures of minerals and each mineral has its own set of physical characteristics. For example, Quartz melts at about 1725 oC at one atmosphere total pressure; in this case, melting is defined as the temperature at which solid and liquid of the same composition are in equilibrium. In general we must specify the pressure in order to state a unique melting point.
If Quartz is mixed with Alkali Feldspar in some proportion (80% feldspar and 20% quartz, for example) melting occurs but not in the same way that the melting of a pure compound occurs. In general, there is no single temperature at which the entire mixture goes from solid to liquid. Rather, there is a range of temperatures at which liquid and solid are present. This is the interval of partial melting or partial crystallization.
Most rocks are mixtures of minerals and each mineral has its own set of physical characteristics. For example, Quartz melts at about 1725 oC at one atmosphere total pressure; in this case, melting is defined as the temperature at which solid and liquid of the same composition are in equilibrium. In general we must specify the pressure in order to state a unique melting point.
If Quartz is mixed with Alkali Feldspar in some proportion (80% feldspar and 20% quartz, for example) melting occurs but not in the same way that the melting of a pure compound occurs. In general, there is no single temperature at which the entire mixture goes from solid to liquid. Rather, there is a range of temperatures at which liquid and solid are present. This is the interval of partial melting or partial crystallization.
1000o C liquid
900o C solid + liquid
800o C solid + liquid
700o C solid + liquid
600o C solid
The amount of liquid decreases as the temperature drops until all of the liquid is used up in producing solids.
 In this hypothetical example partial melting is initiated at about 700 degrees centigrade and completed at 1000 o C. As the temperature increases the amount of solid decreases. Cooling is the reverse. This mixture would be 100% liquid until a temperature of about 1000 o C. Crystallization begins and the amount of solids increase and the amount of liquid decreases as the temperature cools. At about 700 o all of the liquid is gone. The concept of partial melting plays a crucial role in igneous processes. For example, many (most? all?) magmas are formed by partial melting which did not reach the temperature at which all of the material was molten. In general, liquids tend to be less dense than the solids that crystallize from them. In a mixture of crystals and liquids the liquid (less dense) will attempt to migrate upwards whereas the crystals may sink. In other words, this hypothetical mixture need not be heated to 1000 o C to produce a magma. Magma generation is initiated at about 700 o .
In this hypothetical example partial melting is initiated at about 700 degrees centigrade and completed at 1000 o C. As the temperature increases the amount of solid decreases. Cooling is the reverse. This mixture would be 100% liquid until a temperature of about 1000 o C. Crystallization begins and the amount of solids increase and the amount of liquid decreases as the temperature cools. At about 700 o all of the liquid is gone. The concept of partial melting plays a crucial role in igneous processes. For example, many (most? all?) magmas are formed by partial melting which did not reach the temperature at which all of the material was molten. In general, liquids tend to be less dense than the solids that crystallize from them. In a mixture of crystals and liquids the liquid (less dense) will attempt to migrate upwards whereas the crystals may sink. In other words, this hypothetical mixture need not be heated to 1000 o C to produce a magma. Magma generation is initiated at about 700 o .

When magma reaches the surface it is called lava. Magmas which cool at the surface of the Earth are extrusive whereas those that cool within the Earth are intrusive.
Review the relationships between plate boundaries and igneous activity.
 Classification of Igneous Rocks
Classification of Igneous Rocks
Why do we need to classify things? In part because it requires us to focus on attributes of the things that we are interested in and to focus on those that we feel are the most important. In part we classify things because we want to be able to efficiently communicate information. Giving something a name can aid but only if everyone understands the basis for assigning the name in the first place. For example, if your Universe consisted of Fords and Chevrolets and you wanted to devise a classification scheme you would probably not use color as an important attribute.
Two properties of igneous rocks that we will focus on are texture and mineralogy.
Texture refers to the size, shape and arrangement of the grains in the rock.
- phaneritic - coarse grained - you can see the individual crystals
- aphanitic - fine grained - you can't see the individual crystals or grains
- porphyritic - big grains and small grains
- phenocryst - big
- groundmass - small
- phenocryst - big
 In general, igneous rocks have an interlocking texture. As minerals crystallize from a liquid they compete for space. There is a tendency for the minerals to inter grow making for "jagged grain boundaries".
A number of variables control the texture of an igneous rock but, the rate of cooling is certainly important. The more rapid the cooling rate the finer the grain size. When lava at 1000 degrees C pours out on the surface of the Earth it is in contact with the air at about 25 degrees C. Heat flows from high values to low in an attempt to bring the two bodies into thermal equilibrium. Therefore, the lava would cool rapidly and probably develop an aphanitic texture.
In general, igneous rocks have an interlocking texture. As minerals crystallize from a liquid they compete for space. There is a tendency for the minerals to inter grow making for "jagged grain boundaries".
A number of variables control the texture of an igneous rock but, the rate of cooling is certainly important. The more rapid the cooling rate the finer the grain size. When lava at 1000 degrees C pours out on the surface of the Earth it is in contact with the air at about 25 degrees C. Heat flows from high values to low in an attempt to bring the two bodies into thermal equilibrium. Therefore, the lava would cool rapidly and probably develop an aphanitic texture. Consider a body of magma at 800 degrees C which is intruded into country rock which is 400 degrees C. Heat will flow from the magma to the country rock and its temperature will increase. At the margin of the magma chamber the rate of cooling is quite high as compared with the rate of cooling at the center of the chamber. Therefore, we might expect some variation in grain size from the margin to the center of the chamber; finer grained at the margin and coarser toward the center.
Mineralogy - Recall that the most abundant mineral groups in the crust are plagioclase and alkali feldspar. Norman Bowen (about 1915) proposed the following sequences of crystallization of silicates from a magma.
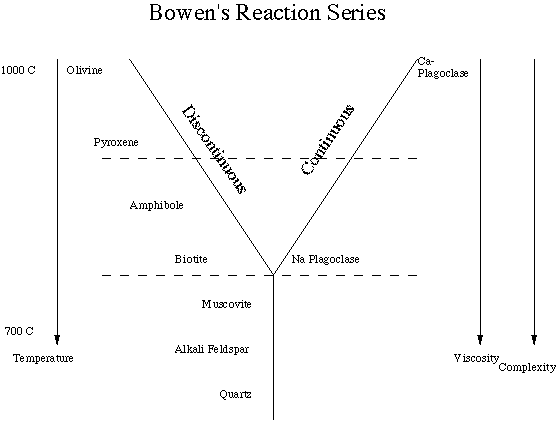
 Several important generalizations can be made based on our knowledge of the behavior of the phases present in the reaction series.
Several important generalizations can be made based on our knowledge of the behavior of the phases present in the reaction series.
- With the singular exception of quartz, the other phases present represent solid solution series.
- The viscosity (resistance to flow) of a melt (magma/lava) increases with decreasing temperature.
- The complexity (amount of sharing of the oxygens of the silicon-oxygen tetrahedrons) increases with decreasing temperature.
- The dashed lines are drawn to reflect three mineral assemblages :
- high temperature - olivine, pyroxene and Ca-rich plagioclase;
- intermediate temperature - amphibole, biotite and Na-rich plagiolase; and
- low temperature - muscovite, alkali feldspar and quartz.
- high temperature - olivine, pyroxene and Ca-rich plagioclase;
- Bowen's Reaction Series points out that there are commonly occurring mineral assemblages (based on similar temperatures of formation/crystallization). For example, quartz and olivine (at least the magnesium-rich variety) are not expected to occur together as an equilibrium assemblage.
- high temperature
- olivine
- pyroxene
- amphibole
- biotite
- low temperature
 He referred to this sequence as the discontinuous side of the reaction series. He determined (by laboratory experiments) that as a magma containing olivine cooled, the olivine would react with liquid (dissolve in the liquid) and the liquid would crystallize a pyroxene. He thought that pyroxene would yield to amphibole which would yield to biotite. He knew that pyroxene melted incongruently to form an olivine and assumed that amphibole and biotite would do the same. The fact that the series does not work exactly as Bowen predicted does not lessen the value of the series as a framework for working with igneous rocks.
He referred to this sequence as the discontinuous side of the reaction series. He determined (by laboratory experiments) that as a magma containing olivine cooled, the olivine would react with liquid (dissolve in the liquid) and the liquid would crystallize a pyroxene. He thought that pyroxene would yield to amphibole which would yield to biotite. He knew that pyroxene melted incongruently to form an olivine and assumed that amphibole and biotite would do the same. The fact that the series does not work exactly as Bowen predicted does not lessen the value of the series as a framework for working with igneous rocks.
Bowen was not aware that biotite, muscovite and amphibole must contain (OH)- or fluorine or chlorine. If one of these volatile components is not present in the melt then these phases will not form.
 At the same time the discontinuous reactions are taking place a continuous reaction is occurring"
At the same time the discontinuous reactions are taking place a continuous reaction is occurring"
- high temperature
- calcium-rich plagioclase
- calcium-sodium plagioclase
- sodium-rich plagioclase
- low temperature
Recall that the plagioclase series is one continuous solid solution series. At high temperatures the plagioclase is rich in calcium, and at low temperatures it is rich in sodium.
At temperatures below the continuous and discontinuous sides of the reaction series the following minerals crystallize:
- high temperature
- muscovite
- alkali feldspar
- quartz
- low temperature
If we focus on the feldspars, we can take advantage of the following relationship in relating the mineralogy of an igneous rock to the temperature it formed at.
- high temperature
- calcium-rich plagioclase
- sodium-rich plagioclase
- alkali feldspar (a solid solution between K and Na feldspars)
- low temperature
Quartz has the highest melting point of the individual minerals in Bowen's Reaction Series but it crystallizes at the lowest temperature from a magma. Thus, the importance of understanding the properties of a mixture.
 Thought Question
Thought Question
- Note that the composition of the melt is important. If melt did not contain any Fe or Mg, which minerals would not crystallize? If the melt did not contain any water, which minerals would not crystallize?
 Finally, we are able to construct a classification scheme for igneous rocks using texture and mineralogy. The temperature will be estimated by the feldspar(s) present and the cooling rate by the texture. In constructing the following chart a number of short-cuts have been taken.
Finally, we are able to construct a classification scheme for igneous rocks using texture and mineralogy. The temperature will be estimated by the feldspar(s) present and the cooling rate by the texture. In constructing the following chart a number of short-cuts have been taken.
Alkali Feldspar Sodium Plagioclase Calcium Plagioclase
Phaneritic Granite Diorite Gabbro
Aphanitic Rhyolite Andesite Basalt
Take a Tour of a Rock Garden and look at some other igneous rocks.
Granite is a coarse grained igneous rock which contains abundant alkali feldspar. Granites also contain quartz. This is a low-temperature assemblage. Rhyolite is the mineralogical equivalent of granite but it formed as a result of rapid cooling giving the rock the fine grained texture. Think about the relationships between Diorite and Andesite and Gabbro and Basalt. Look at the steps leading up to this building from the parking lot. Does it look similar to this picture? The reddish mineral is alkali feldspar and quartz is the gray, glassy mineral. The black material is a mixture of biotite and amphibole; thus, this rock formed in the presence of (OH).
If the rock is a granite but with a porphyritic texture it would be a granite porphyry. It if is a rhyolite but with a porphyritic texture it would be a rhyolite porphyry.
Viscosity is a measure of "resistance to flow". A liquid with high viscosity flows with difficulty. In general, as the temperature of the liquid increases the viscosity of the liquid decreases and the liquid flows more easily.
- Tabular Bodies - relatively low viscosity to allow magma to follow relatively narrow openings.
- Dikes - tabular bodies that cut across the "structure" of the enclosing rock.
- Sills - tabular bodies that are oriented parallel to the "structure of the enclosing rock.
- Laccoliths - bodies that "dome up" the overlying rocks
- Lopoliths - bodies with a bottom that has "subsided" into the underlying rokcs
- Dikes - tabular bodies that cut across the "structure" of the enclosing rock.
- Irregular bodies include stocks and batholiths which typically were formed from highly viscous melts.
 Shapes of Igneous Bodies
Shapes of Igneous Bodies

 You may wish to print a copy of the lecture outline (minus the illustrations) and you have two options:
You may wish to print a copy of the lecture outline (minus the illustrations) and you have two options: