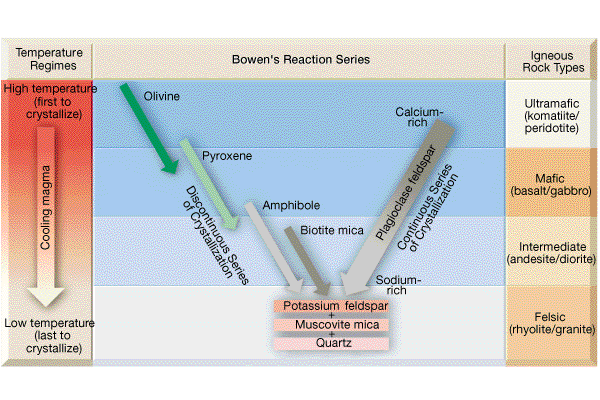
- With the singular exception of quartz, the other phases present represent solid solution series.
- The viscosity (resistance to flow) of a melt (magma/lava) increases with decreasing temperature.
- The complexity (amount of sharing of the oxygens of the silicon-oxygen tetrahedrons) increases with decreasing temperature.
- The dashed lines are drawn to reflect three mineral assemblages :
- high temperature - olivine, pyroxene and Ca-rich plagioclase;
- intermediate temperature - amphibole, biotite and Na-rich plagiolase; and
- low temperature - muscovite, alkali feldspar and quartz.
- Bowen's Reaction Series points out that there are commonly occurring mineral assemblages (based on similar temperatures of formation/crystallization). For example, quartz and olivine (at least the magnesium-rich variety) are not expected to occur together as an equilibrium assemblage.
At high temperatures two sequences exist. One includes those minerals which are rich in Fe and Mg (ferromagnesian):
- high temperature
- olivine
- pyroxene
- amphibole
- biotite
- low temperature
Bowen referred to this sequence as the discontinuous side of the reaction series. He determined (by laboratory experiments) that as a magma containing olivine cooled, the olivine would react with liquid (dissolve in the liquid) and the liquid would crystallize a pyroxene. He thought that pyroxene would yield to amphibole which would yield to biotite. He knew that pyroxene melted incongruently to form an olivine and assumed that amphibole and biotite would do something similar.
The fact that the series does not work exactly as Bowen predicted does not lessen the value of the series as a framework for working with igneous rocks.
Bowen was not aware that biotite, muscovite and amphibole must contain (OH)- or fluorine or chlorine. If one of these volatile components is not present in the melt then these phases will not form.
At the same time the discontinuous reactions are taking place a continuous reaction is occurring"
- high temperature
- calcium-rich plagioclase
- calcium-sodium plagioclase
- sodium-rich plagioclase
- low temperature
Recall that the plagioclase series is one continuous solid solution series. At high temperatures the plagioclase is rich in calcium, and at low temperatures it is rich in sodium.
At temperatures below the continuous and discontinuous sides of the reaction series the following minerals crystallize:
- high temperature
- muscovite
- alkali feldspar
- quartz
- low temperature
If we focus on the feldspars, we can take advantage of the following relationship in relating the mineralogy of an igneous rock to its temperature of formation:
- high temperature
- calcium-rich plagioclase
- sodium-rich plagioclase
- alkali feldspar (a solid solution between K and Na feldspars)
- low temperature
Quartz has the highest melting point of the individual minerals in Bowen's Reaction Series but it crystallizes at the lowest temperature from a magma. Thus, the importance of understanding the properties of a mixture.
Thought Questions
Finally, we are able to construct a classification scheme for igneous rocks using texture and mineralogy. The temperature will be estimated by the feldspar(s) present and the cooling rate by the texture. In constructing the following chart a number of short-cuts have been taken.
Alkali Feldspar Sodium Plagioclase Calcium Plagioclase
Phaneritic Granite Diorite Gabbro
Aphanitic Rhyolite Andesite Basalt
Be able to identify each of the six common igneous rocks with the mineral assemblages that they contain. Be able to describe when you would expect to use the aphanitic name and when you would use the phaneritic name.
The is a rather specific relationship between temperature, plate tectonic setting and type of igneous rock. In general, basalts are found associated with a spreading center, andesites with a subduction zone, and rhyolites with continent-continent collision. However, look at the Hawaiian Islands. They are a long way from the nearest plate boundary or spreading center. Therefore, there must be exceptions to these generalizations.
Take a Tour of a Rock Garden and look at some other igneous rocks.
Granite is a coarse grained igneous rock which contains abundant alkali feldspar. Granites also contain quartz. This is a low-temperature assemblage. Rhyolite is the mineralogical equivalent of granite but it formed as a result of rapid cooling giving the rock the fine grained texture. Think about the relationships between Diorite and Andesite and Gabbro and Basalt. Look at the steps leading up to this building from the parking lot. Does it look similar to this picture? The reddish mineral is alkali feldspar and quartz is the gray, glassy mineral. The black material is a mixture of biotite and amphibole; thus, this rock formed in the presence of (OH).
If the rock is a granite but with a porphyritic texture it would be a granite porphyry. It if is a rhyolite but with a porphyritic texture it would be a rhyolite porphyry.
Viscosity is a measure of "resistance to flow". A liquid with high viscosity flows with difficulty. In general, as the temperature of the liquid increases the viscosity of the liquid decreases and the liquid flows more easily. Water modifies the viscosity of a melt. In general, the greater amount of water dissolved in the melt, the lower its viscosity and the more readily it flows.