LIPIDS |
A.
Fatty Acids (see Table 9-1)
·
Rarely free in nature.
·
Esterified as the major
components in lipids.
·
In higher animals and plants: Predominantly as C16 and C18 species, e.g. palmitic acid (16:0), linoleic
acid (18:2), and stearic acid (18:0). Over half are unsaturated.
·
Most have an even number of C.
·
For most unsaturated FAs, the 1st
double bond between C9 and C10.
·
For polyunsaturated FAs, the
double bonds tend to occur every 3 C atoms.
·
Saturated FAs as individual
molecules are highly flexible. A wide
range of conformation allowed by single bonds between two neighboring
carbons. The fully extended conformation
is the most stable one.
·
Double bonds in FAs are almost
always cis,
with a rigid 30° bend. The reduced van der Waals interactions cause melting point to drop.
B.
Triacylglycerols
·
Triacylglycerols (= Triglycerides)
or neutral fats
·
A major component of the oils and fats in plants and
animals.
·
Triacylglycerols are energy
reservoirs in animals. Less oxidized
than carbohydrates, proteins. Less
hydrated, lighter in weight.
·
Simple triacylglycerols: only one
type of FA
·
Mixed triacylglycerols: 2 or 3
types of FA.
·
Plant oils are usually richer in unsat.
FA residues than are animal fats.
C.
Glycerophospholipids (Phosphoglycerides)
- Major lipid components of biological membranes.
- See Table 9-2 for important examples of glycerophospholipids.
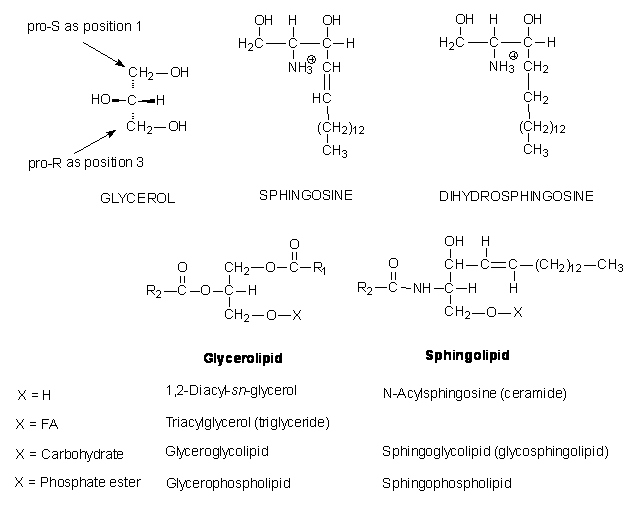
D. Sphingolipids
·
Also major lipid
components of biological membranes.
·
Derivatives of the C18 amino alcohols sphingosine
and dihydrosphingosine.
·
Sphingophospholipids
¨ Sphingomyelins (= sphingophospholipids; see Fig. 9-7)
¯ Ceramides bearing either
a phosphocholine or a phosphoethanolamine.
¯ The most common sphingolipid.
¯ The membranous myelin sheath that surrounds and electrically
insulates many nerve cell axons is particularly rich in sphingomyelin.
·
Sphingoglycolipids
¨ Cerebrosides
¯ The simplest sphingoglycolipid.
¯ The –CH2-OH group is
derivatized by a single sugar residue, such as galactocerebroside
in neuronal cell membranes and glucocerebrosides.
¨ Gangliosides
¯ Most complex, are ceramide
derivatized by oligosaccharides containing at least one sialic
acid.
¯ Primary components of cell surface membranes. Their complex carbohydrate head groups act as
specific receptors for hormones, bacterial toxins, and also function in
cell-cell recognition.
E.
Cholesterol (Fig. 9-10)
·
A major component of animal plasma membranes.
·
Metabolic precursors of steroid hormones (e.g. testostorone, estrogens)
·
Tend to decrease membrane fluidity. Its rigid steroid ring system interferes with
the motions of FA’s side chain.
MEMBRANES AND
FUNCTIONS OF BIOLOGICAL MEMBRANES
Compartmentation
·
Cells use plasma membrane as an envelope.
·
Subcellular organelles –
nuclei, mitochondria, chloroplasts, endoplasmic reticulum, Golgi apparatus,
etc.
Control
of the passage of materials
·
Control the flow of nutrients, waste products, ions, etc.
·
Contain “pumps” and “gates.”
Many
fundamental biochemical processes occur on or in a membranous
scaffolding
·
Oxidative phosphorylation
·
Photosynthesis
Processing
of information
·
Sensory stimuli
·
Intercellular communication
·
Nerve impulses
·
Hormonal actions
ARTIFICIAL MEMBRANES
I. Lipid Monolayer
·
Low
concentration of lipids tend to form a monolayer on
the surface of water.
·
Polar
heads of these amphiphilic molecules (see Fig. 9-4) are
immersed in the water, and the hydrophobic tails extend into the air.
II. Micelles
·
Globular
aggregates whose hydrocarbon groups are out of contact with the water medium.
Single-hydrocarbon-tail
lipids (Fig. 9-13): e.g. soap, detergents.
·
Form
micelles once their concentrations are above the Critical Micelle Concentration
(CMC).
·
For
small single-tail lipids, e.g. dodecylsulfate, CMC » 1 mM.
Double-tail
lipids (Fig. 9-14):
·
Tend to form
flattened micelles, part of them are lipid bilayer in
structure.
·
For most
biological double-tail lipids, CMC < 10-6 M.
III. Lipid Bilayer and Liposomes (Fig. 9-15)
·
Liposomes are closed, self-sealing, solvent-filled vesicles that are
bounded by a single Lipid Bilayer (LB).
·
LBs
are impermeable to most polar substances.
·
Permeabilities of LB increase with solubilities
in nonpolar solvents.
·
LBs
are appreciably permeable to water despite its polarity. Its small size makes water significantly
soluble in the hydrocarbon core of LBs.
·
LBs
are two-dimensional fluids.
Transverse Diffusion: Flip-flop.
extremely slow.
(Fig. 9-16)
Lateral Diffusion: Much faster.
Lipids are highly mobile in the plane of the bilayer. (Fig. 9-16)
IV. Bilayer
Fluidity varies with
·
Lipid
Composition: More fluid with shorter
chains, more unsaturated fatty acids, and less cholesterol.
· Temperature: More fluid above Transition Temperature. (Fig. 9-18)
BIOLOGICAL
MEMBRANES
·
Composed
of proteins associated with a lipid bilayer matrix.
·
Specific
proteins occur only in particular membranes.
I. Peripheral or
Extrinsic Proteins
·
Can
be dissociated from membranes by relatively mild procedures that leave the
membrane intact. e.g.
cytochrome c.
II. Integral or
Intrinsic Proteins (Figs.
9-19, 9-26)
·
Tightly
bound to membranes by hydrophobic forces.
·
Can
only be separated from membranes by treatment with agents that disrupt membrane
structure.
·
All
biological membranes contain integral proteins.
·
Asymmetrically
oriented amphiphilic molecules.
·
No
integral proteins are completely buried in a membrane.
·
For
many integral proteins, the hydrophobic segments anchor the active region of
the protein to the membrane.
·
The transmembrane parts are highly hydrophobic and, in some
cases, are simple alpha helices.
III. Asymmetric
Orientation of Membrane Proteins (Figs.
9-20, 9-26)
·
Some
membrane proteins (integral or peripheral) are located on or exposed to only a
specific surface of a membrane.
·
Other
integral proteins (known as transmembrane proteins)
span the membrane. These proteins are
oriented in only one direction with respect to the membrane.
FLUID MOSAIC
MODEL
·
A
unified theory of membrane structure proposed by Jonathan Singer and Garth
Nicholson in 1972.
·
Integral
proteins resemble “icebergs” floating in a fluid 2-dimensional lipid “sea.”
·
Has
been experimentally verified.
·
Example
1 (Fig. 9-28): Photobleaching
and recovery
·
Example
2 (Fig. 9-27):
¨ Mouse cells –
labeled with mouse protein-specific antibody-green dye conjugate.
¨ Human cells - labeled with human
protein-specific antibody-red dye conjugate.
¨ Both cell types were fused by
treatment with Sendai virus.
¨ Initially, the green and red
colors were polarized on a given cell surface.
After ~ 40 min, the two color were completely
mixed.